Five Unconquered Frontiers for Longevity biotech: Our 2026 Call for Proposals
We're looking for enabling technologies that propel therapeutic asset concepts to have the potential to go on to become civilization-changing medicines
In terms of announcements, it’s been a huge week for our team - from co-leading Aerska’s $39M Series A to leading Loyal’s $100M Series C. From Aerska’s brain-shuttled RNAi therapeutics to what Forbes calls “The Dog Longevity Startup”, the age1 crew is committed to supporting ultra-ambitious founders and the generational biotech companies they craft.
So what’s next?
First, a case study, then a call for proposals.
Aerska’s approach and pace of progress underscore our thesis-driven approach to early-stage biotech investing.
We look for enabling technologies that propel therapeutic asset concepts to have the potential to go on to become civilization-changing medicines.
In the case of neurodegenerative disease, we identified four core challenges:
Crossing the blood-brain barrier
The patients most likely to benefit from therapeutics (prodromal phase, early disease course, etc.) are the ones least likely to go through invasive procedures (stereotactic injections and spinal taps are common). Systemically-administered approaches have been intractable until extremely recently
Intracellular proteinopathies (ubiquitous in neurodegenerative disease) are not accessible to antibodies, whether or not they are brain-shuttled
Target overcrowding
That’s why brain-shuttled RNAi, aimed at early intervention in high-risk cohorts, has the potential to change how we think about neurodegeneration, one of the greatest challenges of our time.
We are laser-focused on the following:
Please reach out if any of the following topics resonate and you have a thesis on how to surmount these challenges.
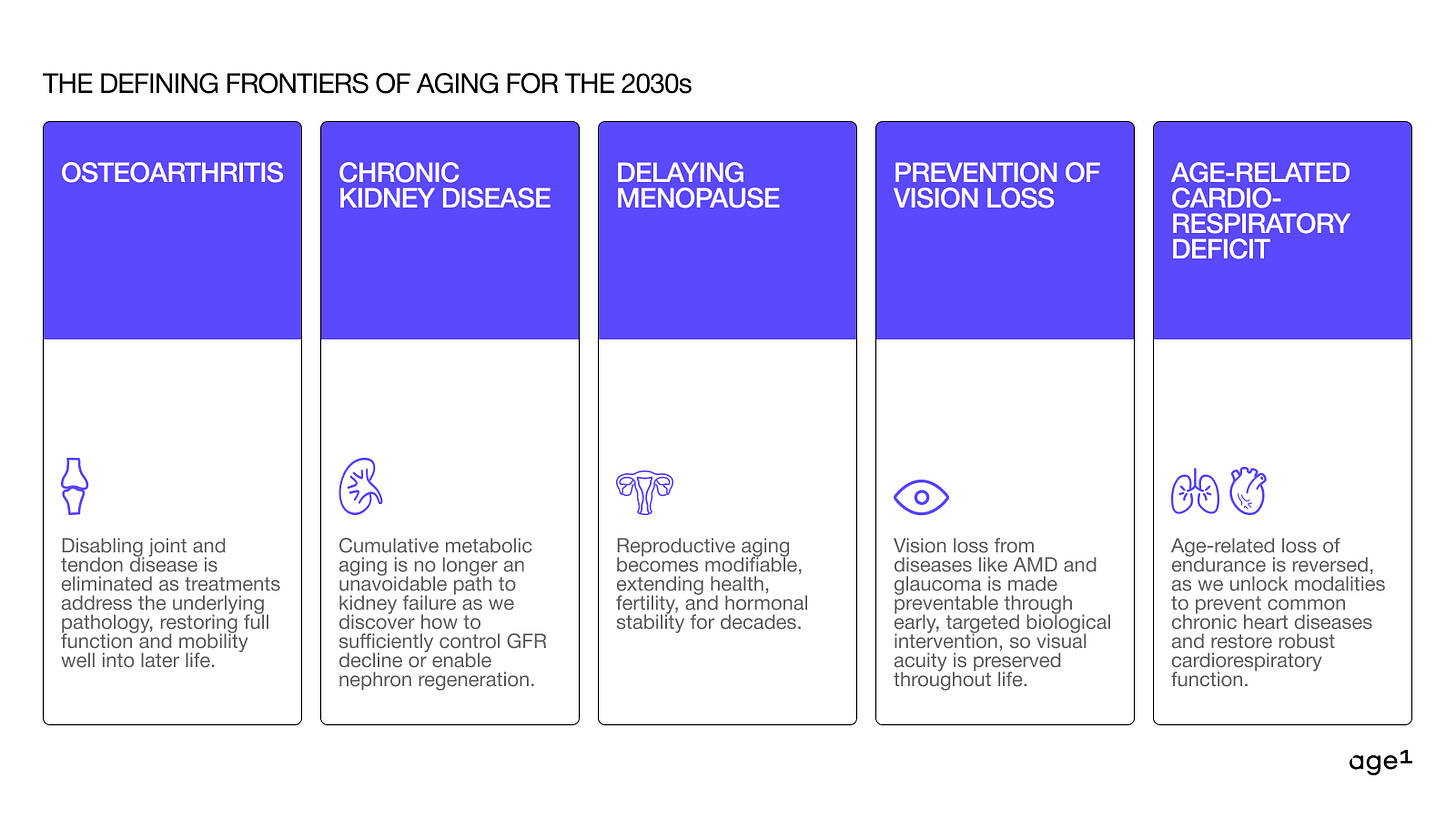
A lot of the time, founders teach us the best spaces to build in, or invent new spaces altogether! While our focus and interests go beyond the five areas listed below, we have a hunch these five will be especially important for the 2030s.
Osteoarthritis (and other approaches for joint/tendon degeneration)
OA is a leading cause of disability and will affect 1B individuals by 2050
Approaches that only improve cartilage without affecting pain and function will have intractable clinical development paths given WOMAC is the primary endpoint (e.g., FGF18); approaches that solely address pain will not address the underlying cause of the disease and may actually accelerate deterioration (e.g., NGF inhibitors).
Cartilage-anchoring protein (e.g., Sanofi’s SAR446959) and siRNA approaches are new to the scene in OA
Chronic kidney disease
The definition of CKD is eGFR < 60 or urine albumin/creatine >= 30. eGFR drops by 1 point per year after age 40, meaning pretty much everyone above a certain age (~70) has CKD (symptomatic, secondary to hypertension or diabetes, or asymptomatic). eGFR and uACR are “integrators” of cumulative metabolic aging.
We never regrow nephrons. Current SOC (standard of care) only affects blood pressure (fluid balance) or blood glucose.
Unlike HbA1c or LDL-C, there is no such thing as “sufficiently controlled” GFR decline
SGLT2 inhibitors are multi-billion dollar drugs but have barely penetrated the asymptomatic CKD market.
Delaying menopause
Prevention of vision loss
MR/GWAS approaches are uncovering many promising targets for wet AMD, dry AMD, glaucoma, etc. Selecting the right modality with tissue specificity is key.
There are no approved drugs for intermediate AMD, despite it being the optimal phase to intervene in (hard to rescue vision loss when it is pretty much 100% gone).
Targets that affect AMD progression may be different than those that affect “the path to AMD” (prevention; subclinical to intermediate AMD)
Pharmacological SOC for presbyopia are eye drops that induce pupil contraction (no modulation of underlying biology: loss of flexibility in the eye’s crystalline lens)
Age-related cardiorespiratory deficit
Cardiomyocytes are post-mitotic and do not regenerate in adult humans
Modalities that enable access to cardiomyocyte-specific and intracellular targets are now here (e.g., siRNA conjugated to TfR1 ligand or antibody) and in development for rare conditions (e.g., PLN cardiomyopathy)
MR/GWAS with cardiomyocyte-specific eQTLs has revealed some interesting causal targets that are interesting for common chronic heart diseases but also for modifier effects in rare/monogenic heart diseases (e.g., dilated cardiomyopathy subtypes)
An extremely common consequence of aging with functional, physical, and socioemotional effects is being “short of breath” (age-related decrease in VO2 max, endurance, fatigue resistance, etc.) We now have MR/GWAS hits suggestive of an effect in preventing CAD but also improving markers of cardiorespiratory function (e.g., FEV1, bronchiectasis)